
Keratin is a family of structural, fibrous proteins. These proteins are the primary components of hair, nails, feathers, claws, hooves, and the outer layer of skin in animals.
There are two main types: alpha-keratin and beta-keratin. Both are found in birds, but the beta-keratin is specific to birds (and reptiles) and is responsible for many of the unique properties of birds.
Specifically, many parts of a bird’s body (feathers, beaks, and claws) are composed of both alpha-keratin and beta-keratin, and the individual mixture of these two keratin types is partly responsible for the huge variety in these body parts.
Alpha-keratins are less common in birds than in mammals. Chemically, these are proteins (large molecules made up of amino acids joined in long chains) that form coiled, flexible structures.
In contrast, beta-keratins are smaller proteins that form rigid, stacked sheets. This makes body parts containing a large proportion of beta-keratins particularly tough and durable.
One subfamily of beta-keratins is the feather-beta-keratin subfamily. Chemically, these keratins have some distinct characteristics resulting in distinct material properties:
- They are comparatively small proteins of only about 100 amino acids
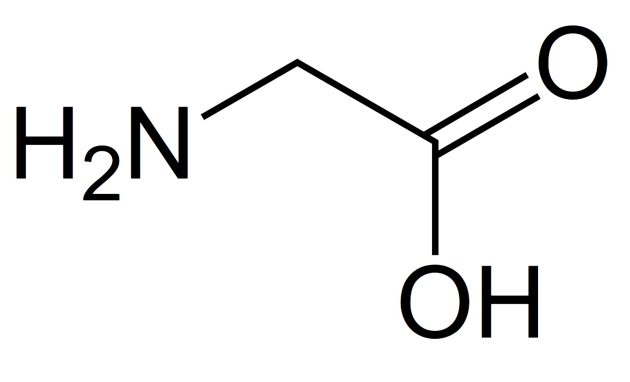
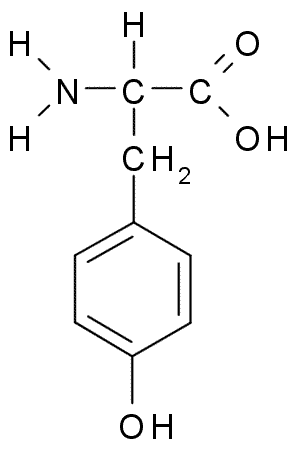
- They contain a core region of about 34 amino acids that is highly consistent across different beta-keratins. This core is rich in two amino acids, glycine and tyrosine, which are a major reason for the rigid structure.
Glycine
Tyrosine
- As mentioned above, the polypeptide chains of beta-keratins are arranged into beta-pleated sheets. These sheets are formed by hydrogen bonds between the carbonyl oxygen of one peptide bond and the amide hydrogen of another, creating a highly stable, folded structure. The sheets then stack together to form strong filaments.
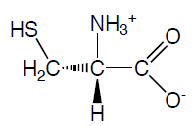
- The stacked beta-pleated sheets are further strengthened by disulfide bridges formed between cysteine units (cysteine is another amino acid). These covalent bonds link adjacent protein chains, creating a highly cross-linked, rigid, and insoluble material. The high density of these disulfide bonds is a key factor in the higher toughness and chemical resistance of avian beta-keratin compared to mammalian alpha-keratin.
Cysteine
- The chemical nature of beta-keratin also contributes to the structural coloration of many bird feathers. The protein self-assembles into intricate nanostructures within feather cells. These structures, along with air cavities, coherently scatter light, producing vibrant, non-iridescent colours like blues and greens. This process is distinct from pigment-based coloration and is directly dependent on the precise arrangement of the keratin and air. It thus requires a highly regular structure that is provided by the beta-keratin.
Interestingly, while feathered non-avian dinosaurs likely produced avian beta-keratin, they lacked specialized feather beta-keratin. This keratin has a higher elasticity and thus is more suited for flight feathers. Thus, this specialized beta-keratin may have played a role in the development of flight.
Illustrations:
- “Cysteine” by Bioreg images is licensed under CC BY 2.0.
- “Feather 1” by treehouse1977 is licensed under CC BY-SA 2.0.
- “Tyrosine” is licensed under CC BY-SA 3.0.
- By Vectorization: Alhadis – Own work based on: Glycine-2D-skeletal.png by Ben Mills, Public Domain, https://commons.wikimedia.org/w/index.php?curid=140657896













Leave a Comment