
As generally in this series, this post will focus on the chemistry involved in avian life, though a bit of poaching from biology may be necessary.
Many aspects of oxygen transport are identical between mammals and birds. So, let’s start with these and later specifically look at the differences between birds and mammals (hint: birds are better at this – but as a bird lover, you probably suspected that anyway).
So, first, the process (biology warning!):
- Air enters the lungs (same in birds and mammals, but the one-way system of birds is more efficient and guarantees higher oxygen levels)
- Oxygen molecules diffuse from the air in the lungs into the blood plasma. Lung tissue has a huge network with extremely fine blood vessels, and the barrier between the air and the blood is very thin.
- From the blood plasma, oxygen diffuses into the red blood cells. The hemoglobin molecules inside the red blood cells bond to up to four oxygen molecules each.
- The red blood cells – now with hemoglobin loaded with oxygen – are being carried by the bloodstream to tissues. There, the oxygen is released.
So, the main chemical reaction here is that between the oxygen and the hemoglobin. It is essentially the same in birds and mammals.
Hemoglobin is a protein that, in its most common form, consists of four polypeptide subunits.
Hemoglobin molecule
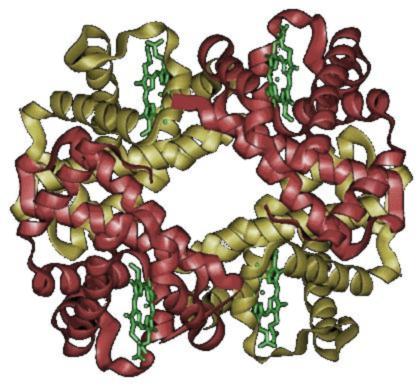
Each of these subunits has one heme group, the part of the hemoglobin that actually binds the oxgen.
Heme unit (same as the four green ones in the hemoglobin molecule above)
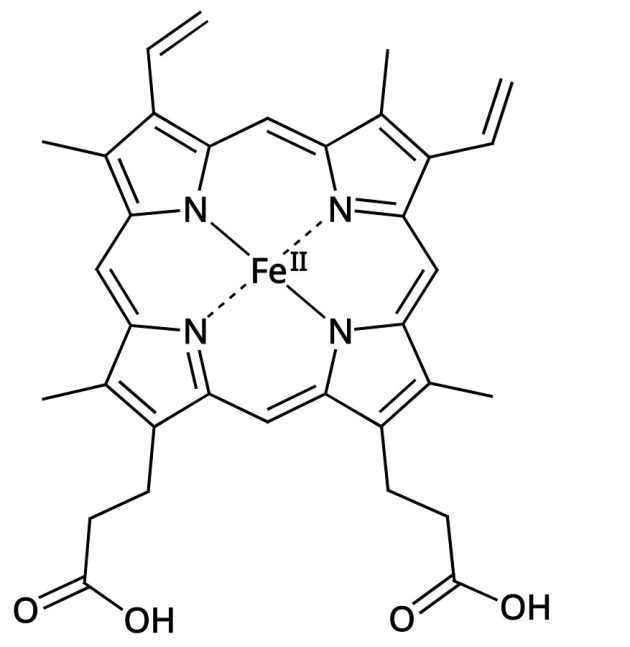
As you can see, the heme units are only a relatively small part of the overall molecule, and the actual oxygen binding sites (the iron atoms) are an even smaller part. Here is a rough breakdown:
The 4 heme units in the hemoglobin molecule account for about 3.8% of the hemoglobin mass and 3.2% of its number of atoms (of which there are about 9300 in total). For the four iron atoms that do the bulk of the actual work, the share is again much smaller – 0.4% of the total mass and only 0.04% of the share of atoms (iron is comparatively heavy compared to the other elements in the hemoglobin molecule).
So, sounds like a rather inefficient arrangement – but one that seems to have survived the test of evolution. And there may be advantages to such a comparatively complex structure. One interesting feature of hemoglobin is that once it has bound one oxygen molecule, it will more easily bind the second, third, and even fourth oxygen molecules. This is because the incorporation of one oxygen molecule changes the shape of the overall hemoglobin molecule, making its other heme groups more accessible.
Now, what is special about the oxygen transport in birds compared to mammals? While the principle is the same, there are some variations that are adapted to the higher metabolic requirements of birds.
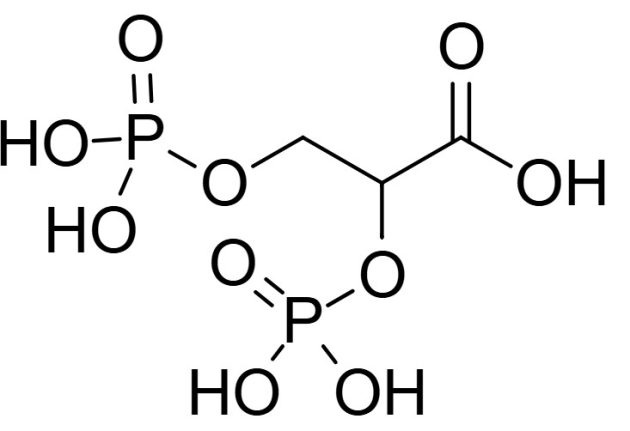
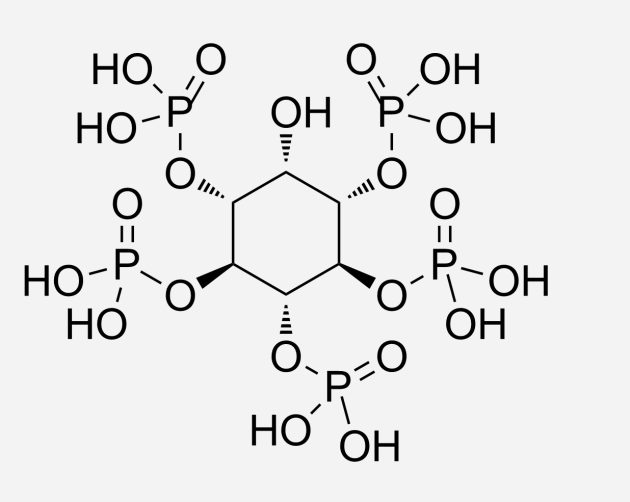
One of these variations is in the somewhat frightening-sounding area of allosteric modulation by organic phosphates. Basically, inside the red blood cells of birds, there are small phosphate molecules. These phosphate molecules can bind to the hemoglobin, which loosens the hemoglobin bond to the oxygen. In turn, this makes it easier for the oxygen to be released from the red blood cells into the tissue. Now, the main phosphate used by mammals is 2,3-bisphosphoglycerate, while in birds, it is inositol pentaphosphate. This phosphate binds more strongly to the hemoglobin and thus is better in driving the oxygen out and into the tissue (which is not exactly the phrasing a proper chemist would typically use, but the principle is correct).
2,3-bisphosphoglycerate
inositol pentaphosphate
Another adaptation is the substitution of some amino acids in the hemoglobin molecule – that is, in the “non-heme” part of the molecule. These changes may also change the strength of the bond between the oxygen and the hemoglobin molecule and thus allow the avian hemoglobin to grab more oxygen than the mammal version.
These adaptations are particularly important in specific situations. One is for embryos. While still in the ovary, the oxygen affinity of their hemoglobin needs to be higher than that of the mother, so they can draw oxygen out of the mother’s bloodstream. Another is for birds such as geese and condors that are regularly flying at very high altitudes, where the oxygen content is low. For example, the Bar-headed Goose has one amino acid changed near the heme group, which makes the oxygen bond stronger.
So, while birds use a similar mechanism to us mammals, they do it better. No wonder they can fly and we cannot.
Photo: Bar-headed Goose, Qinghai Lake, August 2021
Illustrations: “Hemoglobin” is licensed under CC BY-SA 3.0.













Amazing. The avian chemistry of oxygen is so effective. Would the non-avian dinosaurs have had the same? I’d guess so…