
There are two main ways for bird feathers to be colorful. One is based on pigments, one on structure.
With some justification, it can be said that the latter is not really chemistry but rather physics. So, I will focus on pigment-based coloration, but also want to briefly explain structural coloration first.
Structural coloration in bird feathers comes from the physical interaction of light with nanostructures in the feathers and their components (mainly keratin, melanin, and air), not from pigments. Different mechanisms create strong and often iridescent colors:
- Thin-film interference happens when layers of materials with different refractive indices (e.g., keratin and air) reflect light. Some wavelengths then reinforce each other while others cancel each other out. This results in shiny colors that change with the viewing angle. The changing colors of a hummingbird’s or sunbird’s throat are an example.
- Coherent scattering happens when nanostructures (for example, air-filled holes in keratin) scatter light in-phase, so that only certain wavelengths are amplified. This results in matte but vivid colors, often blues and greens, for example, in the blue of a Blue Jay.
- Highly ordered nanostructures act like photonic crystals, reflecting specific wavelengths, which results in precise angle-dependent colors. Starlings are a good example.
These structural colors can be more intense and durable than pigments, and can also change as the structure of the feathers changes (think of the Starling’s feathers only getting its shiny colors once these start wearing down). Furthermore, they can be less costly from an energy standpoint, as they do not require the production or collection of any specific additional chemical substances.
Still, only about 20-30% (though this is a rough estimate) of feather colors are purely structural. Another 10-20% are mixed (for example, green feathers as the result of blue structural coloring and yellow pigment), while the majority (maybe 60-70%) of colors are pigment-based.
Pigment-based color means the color comes from the individual molecules absorbing some light wavelengths. So, the color resulting from this absorption depends on the individual molecule – and here, we are clearly in the realms of chemistry.
Four groups of pigments are somewhat common in bird feathers, starting with the most common and ending with the least common:
- Melanins: blacks, browns, grays
- Carotenoids: reds, oranges, yellows
- Porphyrins: reddish-browns, fluorescent pinks/greens under UV
- Psittacofulvins (in parrots): red/yellow pigments
How about their chemistry?
Melanins (yes, there are several of them – nothing is ever simple) are produced by birds internally via the oxidation and subsequent polymerization of tyrosine, an amino acid. The resulting polymers are insoluble in water and most solvents, have variations in their structure (meaning they can be somewhat undefined), and have aromatic rings with delocalized electrons, which makes them good at absorbing different wavelengths and thus gives them their dark color.
An Eumelanin structure
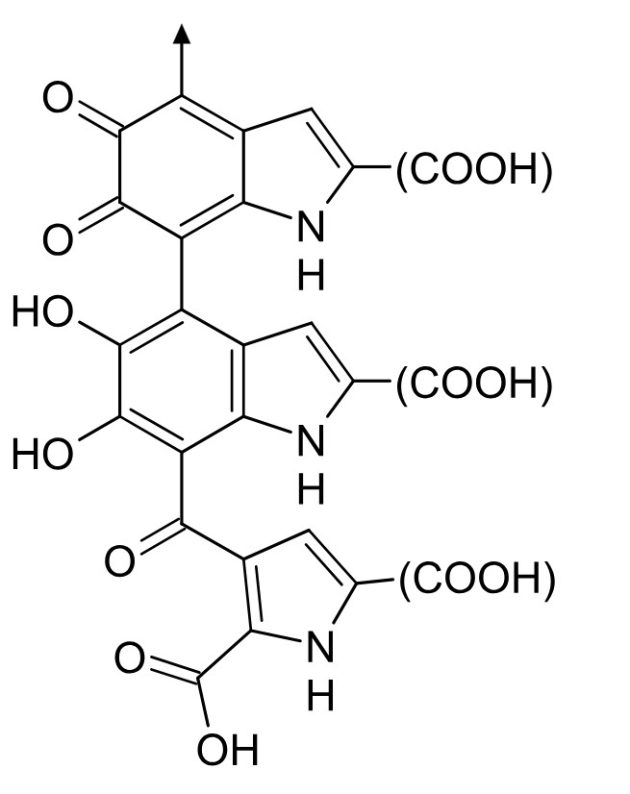
The fact that these melanins can be produced from materials already available to birds via their normal nutrition makes them relatively cheap energetically. Another advantage is that melanins also stiffen feathers, make them more abrasion-resistant, and more resistant to bacteria.
So, that makes melanins very useful pigments – but their color range is limited, and their relative ease of production makes them less suitable as expressions of good health (important in finding a mate). Carotenoids are much better suited to this function.
Beta-Carotin
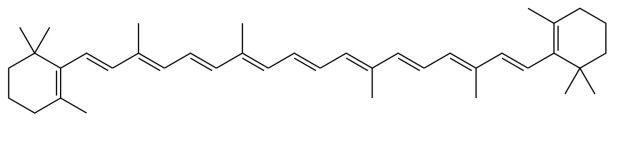
Carotenoids are much more costly for birds to acquire, as they cannot synthesize them internally but have to get them from insects, fruits, or plants. For example, Common Green Magpies may turn blue (from green) if lacking carotenoids in captivity.
In addition, carotenoids have other useful functions for birds, strengthening their immune systems and functioning as antioxidants. So, showing the color of carotenoids in its plumage is a way for a bird to showcase its high level of resources.
All carotenoids are tetraterpene derivatives, so they contain 40 carbon atoms and are based on 8 isoprene units. They are highly unsaturated with conjugated double bonds, enabling them to absorb light of different wavelengths (generally 400 to 550 nm, or violet to green light, resulting in yellow, orange, or red color) depending on the detailed chemical structure.
Some carotenoids are utilized directly as pigments by birds; others are first converted into different, but still related chemicals with different colors and properties.
Porphyrins are much rarer pigments in birds than melanins and carotenoids. Chemically, they are organic compounds based on a cyclic tetrapyrrole: four pyrrole rings connected in a ring, forming a continuous aromatic system.
Basic Porphyrin structure
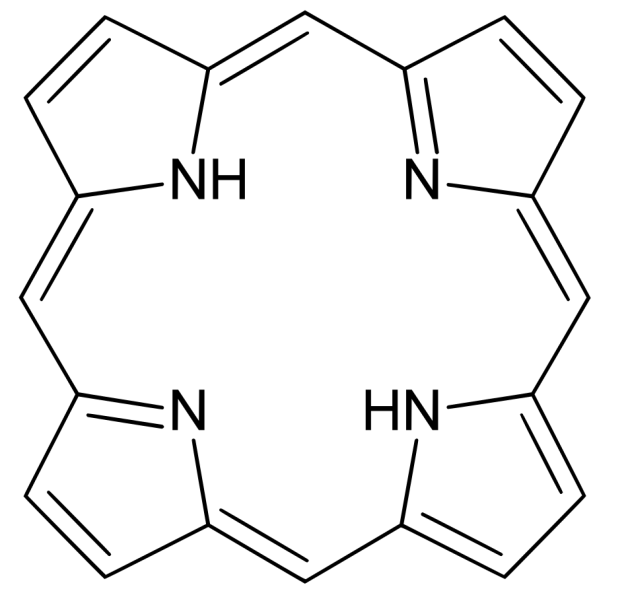
Birds can synthesize porphyrins themselves. They use them to achieve colors including red, brown, green, and pink. Some porphyrin colors fluoresce under UV light (which is invisible to humans but visible to some birds). Another interesting aspect is that these colors are not very stable under direct sunlight, limiting their use to areas such as underwings.
Finally, psittacofulvins can be thought of as the answer of the parrot family to carotenoids – psittacofulvins have similar colors (red, orange, yellow) but are only used by true parrots, and have a completely different chemical structure. They are linear polyenes terminated by an aldehyde group, with the length of the polyene chain as the difference between the individual psittacofulvins. These pigments are produced by the parrots in their growing feathers.
Generic formula of Psittafulvin
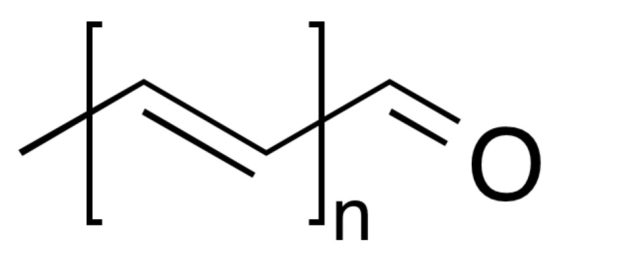
Many birds use both structural and pigment-based coloration at the same time. For example, in parrots, the blues and greens are structural while the reds and yellows are based on the pigment class just mentioned, the psittacofulvins.
Source:
- Psittacofulvin
- Porphyrin
- Carotenoid
- Melanin
- Green Magpies turning blue
- Bird Coloration
- Melanin structure: By Roland Mattern – Roland1952, Public Domain, https://commons.wikimedia.org/w/index.php?curid=6943464
- Beta-Carotin structure: By NEUROtiker – Own work, Public Domain, Link
Photo: Common Green Mapgie, Hongbenghe, Yunnan, December 2023












That eumelanin structure is a thing of beauty – very complex way of making something dark. Great article.
Thanks – once again adding depth to my shallow knowledge ?
Thanks! More to come – just wrote part 14 today.
That means: From spongy zone (Blue-Green) light are reflected.
at cortex psittacine pigment absorb (Blue), and let Green to pass.
Finally we can see Green colored feathers.
because: Pigments absorb light, and yellow is complement with blue, that’s why it absorbs it