
On June 23, 2000, the bulk ore carrier MV Treasure sank between Robben Island and Dassen Island on the coast of South Africa, releasing ~1,300 tons of oil right in the middle of the world’s largest African Penguin colonies. The oil coated tens of thousands of penguins.
At that time, the media showed volunteers washing penguins with warm water and detergent, long lines of blue tubs with birds, and penguins recovering in swimming pools. Even a book was written about this. Why the fuzz? Are oil spills really so dangerous to birds?
Unfortunately, yes. Here is why – as usual for this series, mainly from a chemical (well, yes, also physical, but physicists are usually too busy to invent nuclear power to bother with this kind of stuff) point of view.
Crude oil, such as that released by the MV Treasure, is composed of hydrocarbons, which are hydrophobic, meaning they repel water (not as bad as being homophobic, but not really much better for the birds). More importantly, these hydrocarbons are lipophilic, meaning they like to stick to nonpolar substances. Unfortunately, a bird’s feathers and skin contain exactly the kinds of natural oils and lipids these hydrocarbons love to bind to. So, why is that a problem?
Oil destroys the chemistry of waterproofing
Feathers stay waterproof partly because they are coated in a thin layer of preen oil. The molecules in crude oil mix easily with this natural coating, dissolving it and clogging the feather structure. The feathers then soak with water (they are no longer waterproof), which means they lose their insulating function, resulting in hypothermia and loss of buoyancy.
Toxic hydrocarbons infiltrate the bird’s body
Petroleum contains polycyclic aromatic hydrocarbons that easily dissolve in fat, allowing them to move into biological tissue. Once inside, the polycyclic aromatic hydrocarbons can damage the liver, kidneys, immune system, and even red blood cells. Some are mutagenic or carcinogenic.
An example of a polycyclic aromatic hydrocarbon (public domain). Note that the molecule is polycyclic (i.e., has several cyclic components), is aromatic (each of the rings with three double bonds is an aromatic ring), and is a hydrocarbon (i.e., is composed of hydrogen and carbon)
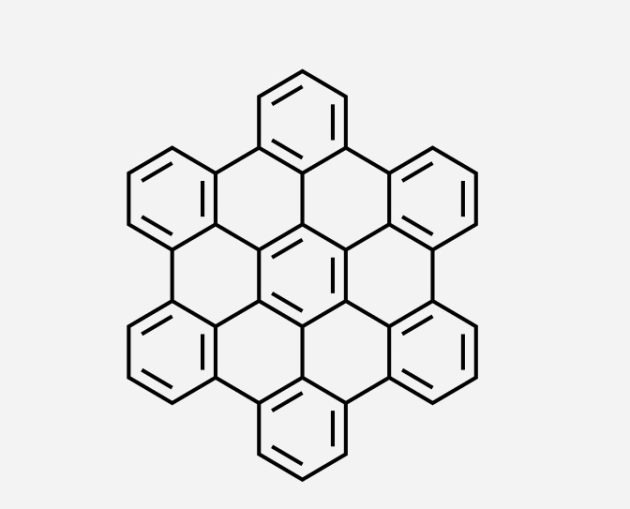
Lighter oil components act like solvents
Refined oil products (like gasoline) are volatile organic compounds—small, highly mobile molecules that behave a bit like paint thinner. These can strip lipids from skin and mucous membranes, causing irritation, dehydration, and further weakening of the bird’s natural barriers. If you have ever had your hands come into contact with paint thinner and observed your skin afterwards, you will understand why this is bad.
Weathered oil becomes even stickier
Finally, as oil weathers in the sun and waves, some of its components break down and oxidize into compounds that behave almost like crude surfactants. These lower the surface tension of water and make it even easier for water to soak into damaged feathers.
Image: African Penguin (not affected by an oil spill, fortunately)













The scientific tone of this post really conveys the misery inflicted by these spills better than any emotional piece would have.